Branching, in nature and neurons
- Nov 13, 2023
- 8 min read
Have you ever stopped to marvel at the intricate patterns found in trees during autumn? As a neurobiology student, I can't help but notice the striking resemblance between the branching patterns of trees and those found in the human brain. But it's not just trees that exhibit these mesmerizing branching patterns; we can see them all around us, from the delicate branching structures of fungi to the intricate formations of river deltas. It's truly remarkable how often these patterns occur in nature, and it's a testament to the beauty and complexity of the world around us.
The vertebrate brain is known to be responsible for intricate decision-making processes, learning, memory, and various other cognitive functions. Such complex activities necessitate a highly extensive and dense interconnection of individual neurons. This is achieved through the formation of multiple synaptic associations, facilitated by elaborate arborizations of axons and dendrites. Therefore branching of neurons has been a study of major light since quite some time. Various pathophysiological conditions are associated with hyper and hypo branching.
The intricate regulation of branching in neurons has captivated the attention of many investigators. Despite being post mitotic cells, neurons have the remarkable ability to regenerate in the case of mechanical damage. However, the precise mechanisms underlying this phenomenon remain elusive.
To gain a deeper understanding of neuronal branching, researchers have conducted both in vitro and in vivo studies. While in vivo studies can be challenging to monitor, in vitro studies are limited by their simplified environment. Given these limitations, is it possible to draw inferences from analogous branching phenomena seen in nature and extrapolate them to neurons?
In this article we would first see if this is possible by taking a reductionist approach. We would first discuss the why aspect and then go to the how aspect in our examples, and try to compare it to neuron cells. Further, based on the inferences gained from studies conducted so far, we would discuss the branching aspect of neurons.
First, take the example of trees.
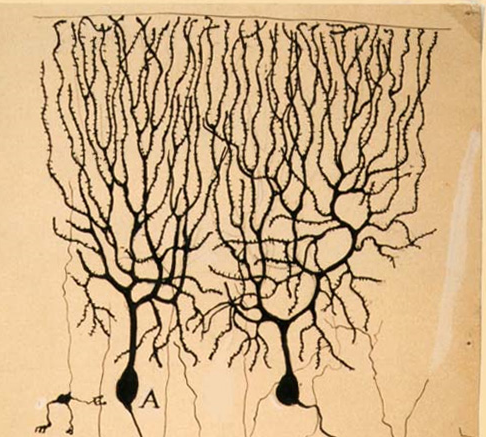
Fig. Left - purkinje cells by Rmoni Cajal, Right- A purkinje like tree in IISER Pune
To begin with the fundamental inquiry, it is crucial to explore the underlying rationale. Trees, as autotrophic organisms, rely on photosynthesis to generate energy, whereby solar radiation serves as the primary stimulus for the electron transport system in photosynthesis. Thus, the evolutionary adaptation of trees emphasizes maximizing the surface area for light absorption to ensure optimal growth and development. In terms of the proximate inquiry, that is, the mechanism through which this process occurs, branches in trees emerge from buds. The apical meristem continuously produces the hormone auxin, which impedes the formation of branches. As the tree grows, and the apical meristem moves further away, the concentration of auxin is substantially reduced, prompting the initiation of branching. This phenomenon is also observed in the formation of sub-branches.

Fig.- Schematic of branching in blood vessel. Source- Internet
Next getting on to blood vessels. Human body has billions of cells. Hence to facilitate abundant gaseous exchange to each and every cell, blood vessels need to have very dense branched morphology. Coming to the how part of the question, it is mediated by notch signaling inhibition which leads to sprouting and inhibition. The notch pathway controls germination and the growth pattern of branches by affecting how the tip cells of blood vessels differentiate, move, and multiply.

Fig.- Colorado river delta. Source- Internet
In the case of river deltas, branching is observed as a consequence of distributaries. This phenomenon occurs when a stream gradually makes its way towards and converges with a lake or ocean. Over time, due to a variety of factors such as the deposition of sediments and uneven terrain, the stream begins to adopt the path that offers the least resistance. This process eventually results in the formation of a bifurcated morphology and a non-linear path. The underlying principle at work here is the principle of least resistance, where the stream naturally follows the path that offers the least resistance in order to achieve its ultimate goal of converging with the larger body of water.
The inherent property of “branching morphogenesis” has been central to mathematicians since long time. A recent nature publication by Dr.Ben Simons in collaboration with Dr Jacco van Rheenen discusses the branching in mammary gland, where they show the probability of termination of a branch and continuation of further branching being statistically equal. Although there being a huge collective decision making, Professor Simons claim it to be no different than flipping a coin. Their prime conclusion was that, although branching might seem to be a very complex and involved process, there exist very simple rules governing the machinery. This comes from their observation that mammillary ducts seem to have very little to no cross over between the branches, rather they seem to fill the empty space while dividing. Hence, they arrived at the hypothesis that the ducts were expanding and separating, but once a tip made contact with another branch, growth would cease. Even though this study was from taking inferences from human mammary glands, the researchers were able to demonstrate the same principles that governed the embryonic development of the mouse kidney, pancreas, and human prostate. Below mentioned link shows the simulation discussed above.
https://www.youtube.com/watch?v=1BmXwOaKwro
Neurons need to process large amounts of sensory inputs. As mentioned above, such a circuitry requires an astounding number of synaptic connections. But it's not just about quantity - precision is also key. Neurons rely on a multitude of inhibitory connections to refine their outputs and ensure that the information they transmit is accurate and reliable. From the above discussed examples, they all rely on branching as a means of optimizing their functionality. In the example of trees, it was about maximizing light exposure. For blood vessels, it is about maximizing the connection to most of the cells. Lastly coming to river delta, it is about following the path of least resistance. However, despite the similarities in their branching structures, each of these systems relies on different mechanisms to achieve their respective functions. The findings of Dr. Simons reveal a fascinating insight into the intricate mechanisms of branching in mammary systems. However, when it comes to neuronal systems, it is important to note that extrapolating mechanisms from other systems may not be practical. This is because the complexity of neural connections cannot be reduced to simpler systems, such as those found in the kidney or pancreas. In fact, it may be impossible to find an analogous system that is as intricate and elaborate as the nervous system. But studying other systems definitely provides a border perspective to the phenomenon of branching.
Neural branching functions are critical for the development of complex cognitive characters. In particular, the localization and timing of neuron cells are of utmost importance. To discuss neural branching, this paper will focus on axonal collateral branching and axonal growth cone guidance. Neuron branching is both intrinsically and extrinsically controlled, and proper and precise branching requires coherent synchronization of both extrinsic and intrinsic orchestration.
Various ligands, such as brain-derived neurotrophic growth factors (BDNF) and neurotrophic growth factors (NGF), are present in gradients to act as guidance cues. These ligands bind to specific receptors, such as Tyrosine Kinase A (TrkA) and Tyrosine Kinase B (TrkB) receptors. However, the gradients of these ligands are subject to change over developmental timelines, and the expression of the corresponding receptors is also subject to change for a given neuronal cell.
Hence an intrinsically choreographed chain of events is required.
First is the local environment must be specified to have the neurons expressing the correct correlate of receptors and the correct correlate of guidance cues
There needs to be proper localization of guidance cues to the corresponding receptor protein complexes vesicles that also should be localized to the corresponding area of growth i.e., the growth cones.
The downstream signaling cascades must be ready (This refers to the accurate secondary signaling elements should be in required phosphorylated states)
Upon binding of the ligands to the receptors, certain downstream signaling cascades relay this message leading to changes in gene expression and other regulatory modifications, which ultimately lead to required change in cytoskeletal organization.
Collateral branching in the axon are the de novo formation of perpendicular projections to the main axon. NGF ligands upon binding to trk (tyrosine kinase) receptors dimerize the receptors, activating further downstream pathways. This further leads to local formation/recruitment of an actin patch which ultimately leads to a filopodial protrusion, ultimately forming a synapse to the target neuron.

Fig- A confocal micrograph of an axonal branch having collateral branches. Acquired in IISER Imaging facility
Axonal bifurcations are also mediated by means of certain extracellular moieties which are localized at specified bifurcation sites. Growth cones upon exposure to such elements end of dividing into two branches. C-type natriuretic peptide (CNP; also known as NPPC) is one such element.

Fig.- Daniel A Gibson and Le Ma et.al 2011
The above mentioned represents the 3 common types of branching seen in axons.
The process of developmental maturation is characterized by a significant increase in the number of branching projections that extend to specific targets. These excess projections subsequently undergo sensory refinement, which facilitates the retrieval of supernumerary projections. A plethora of intriguing studies have been conducted to demonstrate the aforementioned phenomenon.
Now that we know about how neuronal branching is regulated in such complex and intricate manner, let’s see what can go wrong if say for there is over branching.
One possible means to increase branching is through the cessation of synaptic refinement. Mouse models have been utilized in studies that demonstrate that the discontinuation of synaptic refinement results in a decrease in cognitive abilities. Dr. Smith's research has yielded similar findings, where mice that do not undergo synaptic refinement exhibit an aptitude for grasping spatial locations, yet display an inability to subsequently learn new locations even following initial acquisition. Thus, a compelling body of evidence emerges indicating that an excess of synaptic connections can negatively impact learning ability.
In patients afflicted with epileptic seizures, a noteworthy phenomenon is observed wherein there is an augmentation in neural branching, a characteristic that is commonly observed in both human and mouse models. Notably, this phenomenon is accompanied by an increased frequency of bursts, which serves as a significant disease phenotype in this condition. Moreover, heightened firing activity in a neuron induces the formation of brain-derived neurotrophic factor (BDNF) like ligands, which further promote an increase in branching. Consequently, this cascade of events culminates in a greater number of neurons exhibiting elevated branching and firing activity, ultimately leading to the manifestation of the disease phenotype.

Fig- A confocal micrograph of protruding growth cone. Acquired in IISER Imaging facility
Although last years have witnessed immense discoveries in the field, out core understanding for neural branching is still fragmented. Branching might seem very random and insignificant, as discussed above it is a very complex phenomenon and holds non trivial consequences if not mediated properly. Future investigations in this field is definitely going to sharpen about the very fundamental phenomenon of branching. Because to cater the function structure holds a crucial role.
In conclusion, the recent surge of discoveries in the field of neural branching has shed light on the complexity of this fundamental phenomenon. However, our current understanding remains fragmented, and there is a pressing need for further investigations to fully comprehend the intricate mechanisms underlying neural branching. While branching may seem random and insignificant, its non-trivial consequences underscore the critical role it plays in determining neural function and structure. As such, it is imperative that future studies focus on elucidating the fundamental principles that govern branching, as these insights will undoubtedly pave the way for the development of more effective strategies to modulate neural connectivity and function.
REFERENCES
Gibson DA, Ma L. Developmental regulation of axon branching in the vertebrate nervous system. Development. 2011 Jan;138(2):183-95. doi: 10.1242/dev.046441. PMID: 21177340; PMCID: PMC3005597.
Bromfield EB, Cavazos JE, Sirven JI, editors. An Introduction to Epilepsy [Internet]. West Hartford (CT): American Epilepsy Society; 2006. Chapter 1, Basic Mechanisms Underlying Seizures and Epilepsy. Available from: https://www.ncbi.nlm.nih.gov/books/NBK2510/
O'Donnell M, Chance RK, Bashaw GJ. Axon growth and guidance: receptor regulation and signal transduction. Annu Rev Neurosci. 2009;32:383-412. doi: 10.1146/annurev.neuro.051508.135614. PMID: 19400716; PMCID: PMC4765433
Scheele, C et al. Identity and dynamics of mammary stem cells during branching morphogenesis. Nature 542, 313-317 (2017); DOI: 10.1038/nature21046
Scheele, C et al. Identity and dynamics of mammary stem cells during branching morphogenesis. Nature 542, 313-317 (2017); DOI: 10.1038/nature21046
Seybold H, Andrade JS Jr, Herrmann HJ. Modeling river delta formation. Proc Natl Acad Sci U S A. 2007 Oct 23;104(43):16804-9. doi: 10.1073/pnas.0705265104. Epub 2007 Oct 16. PMID: 17940031; PMCID: PMC2040410.
Cardozo PL, de Lima IBQ, Maciel EMA, Silva NC, Dobransky T, Ribeiro FM. Synaptic Elimination in Neurological Disorders. Curr Neuropharmacol. 2019;17(11):1071-1095. doi: 10.2174/1570159X17666190603170511. PMID: 31161981; PMCID: PMC7052824.
About the author
Author: Asutosh Routa
Editor: Asutosh Routa